Codeine and CYP2D6 Ultrarapid Metabolizers: What You Need to Know About Overdose Risk
Codeine Risk Assessment Tool
Assess Your Risk
This tool estimates your risk of codeine overdose based on ethnicity and age. Data from FDA and CPIC guidelines.
Codeine is a painkiller and cough suppressant that sounds harmless-until it isn’t. For most people, a standard dose works fine. But for a small group with a specific genetic trait, even one pill can be deadly. This isn’t a rare edge case. It’s a well-documented, preventable tragedy that’s been known for over a decade. And yet, many doctors still prescribe it without checking a patient’s genetics.
How Codeine Turns Into a Deadly Substance
Codeine itself doesn’t relieve pain. It’s just a placeholder. Your body has to turn it into morphine to get any effect. That transformation happens thanks to an enzyme called CYP2D6. Most people have one or two working copies of the gene that makes this enzyme. Their bodies convert codeine slowly and safely.
But some people-about 1 to 7% of the population, depending on ethnicity-have extra copies of that gene. These are called ultrarapid metabolizers. Their CYP2D6 enzyme works so fast it turns codeine into morphine like a factory on overdrive. Within hours, their blood morphine levels can spike far beyond what’s safe. That’s when breathing slows down. Then stops. And sometimes, the person never wakes up.
The FDA reviewed 64 cases of serious harm from codeine between 2000 and 2013. Of those, 24 ended in death. Twenty-one of those deaths were in children under 12. In nearly every case where genetic testing was done, the child was an ultrarapid metabolizer. One 15-month-old died after a routine tonsillectomy. The dose they were given? Standard. The outcome? Fatal.
Who’s at Risk-and Why It Varies by Ethnicity
This isn’t random. Your risk depends on your ancestry. In North African and Ethiopian populations, up to 29% of people are ultrarapid metabolizers. In Europeans, it’s 3 to 7%. In East Asians, it’s only 1 to 2%. In Australia, about 3% of people fall into this high-risk group.
That means if you’re from North Africa or the Horn of Africa, your chance of a dangerous reaction to codeine is nearly ten times higher than someone from East Asia. Yet, most prescriptions don’t ask where you’re from. They don’t ask about your genes. They just hand out the same pill, the same dose, to everyone.
And it’s not just children. Adults get codeine too-for tooth pain, back pain, after surgery. A 2013 case report described a 28-year-old woman who went into respiratory arrest after taking codeine for postpartum pain. Her genetic test came back positive for ultrarapid metabolism. She survived, but barely.
What the Experts Say-And What They’re Doing About It
The Clinical Pharmacogenetics Implementation Consortium (CPIC), a group of top pharmacologists and geneticists, has been clear since 2012: codeine should not be used in ultrarapid metabolizers. Their 2020 update doubled down, saying the same goes for tramadol, another opioid that turns into a more powerful drug via CYP2D6.
The FDA issued a boxed warning in 2013-the strongest kind. It says codeine can cause death in children who are ultrarapid metabolizers after tonsil or adenoid surgery. The label now says it outright. No guessing. No ambiguity.
Doctors in New Zealand, Australia, the U.S., and Europe have all received these warnings. Medsafe in New Zealand echoed the FDA’s 2013 alert. The European Medicines Agency banned codeine for kids under 12 in 2015. And yet, codeine is still sold over the counter in some countries for coughs. In others, it’s still prescribed for mild pain.
Dr. Mary Relling of St. Jude Children’s Research Hospital predicts codeine will become a drug of historical interest within the next decade. That’s not hyperbole. It’s based on data. Since 2013, pediatric codeine prescriptions in the U.S. dropped by half. More hospitals are starting to test for CYP2D6 before prescribing.

What You Should Do Instead
If you’ve been prescribed codeine, ask: Is there a safer option?
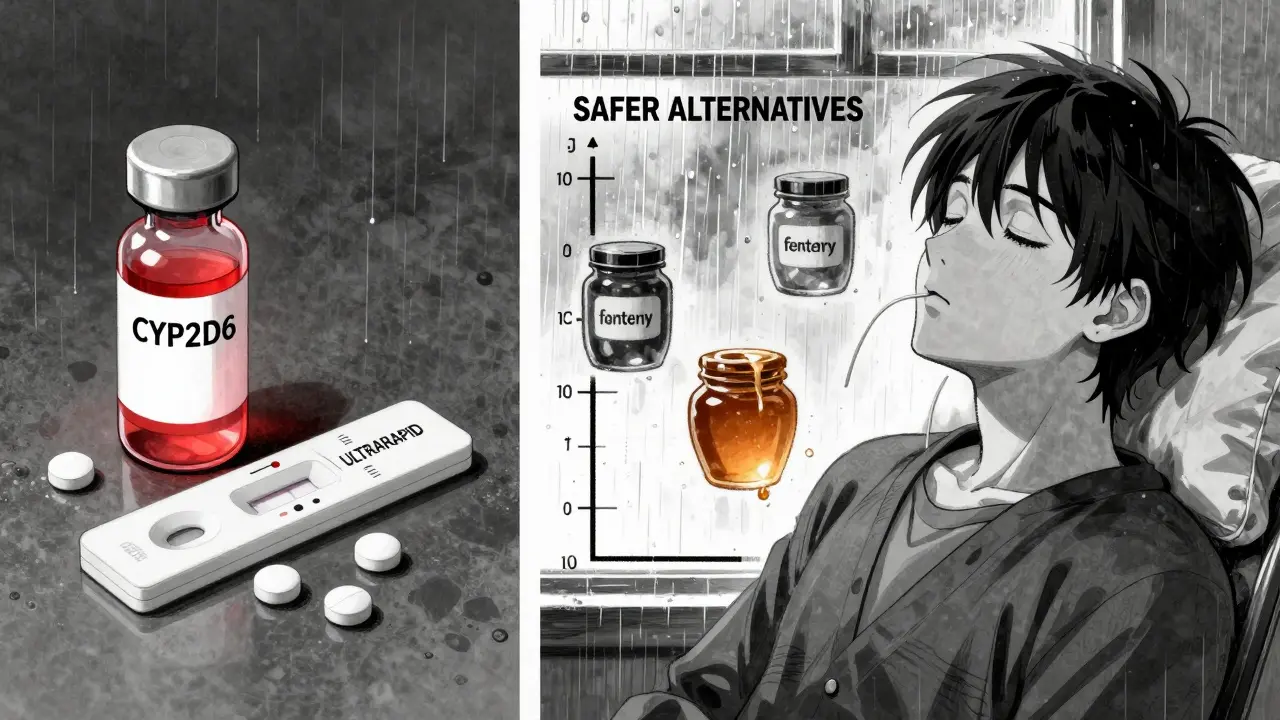
For pain, morphine, hydromorphone, and fentanyl don’t rely on CYP2D6. They work directly. No conversion needed. No genetic surprise. For coughs, dextromethorphan or non-opioid options like honey or guaifenesin are just as effective and far safer.
Even oxycodone and hydrocodone aren’t perfect-they’re partially metabolized by CYP2D6 too. So while they’re better than codeine, they’re not risk-free for ultrarapid metabolizers. The safest bet? Stick to drugs that bypass CYP2D6 entirely.
And if you’re considering genetic testing? It’s worth it. A CYP2D6 test costs between $200 and $500. Insurance may cover it, especially if you’ve had a bad reaction to opioids before. Results take 3 to 14 days. But knowing your status could save your life-or your child’s.
What to Watch For-Signs of Codeine Toxicity
If you or someone you know takes codeine and starts acting unusually, don’t wait. Symptoms of morphine overdose include:
- Extreme drowsiness or trouble staying awake
- Difficulty waking up, even when shaken
- Slow, shallow, or irregular breathing
- Lips or fingernails turning blue
- Cold, clammy skin
- Unresponsiveness
These signs can show up within an hour of taking codeine. They’re not subtle. They’re not "just feeling tired." They’re medical emergencies. Call for help immediately.
Why This Still Isn’t Routine
Here’s the uncomfortable truth: Most doctors don’t test for CYP2D6. Why? Because it’s not standard. Because it takes time. Because labs aren’t always fast. Because many electronic medical records don’t flag genetic risks.
Only about 15 to 20% of major U.S. hospitals have integrated pharmacogenetic testing into their prescribing workflows. In New Zealand, it’s even less common. The technology exists. The guidelines exist. The evidence is overwhelming. But the system hasn’t caught up.
Point-of-care tests are being developed-some labs are testing prototypes that could give results in under two hours. But until then, the burden falls on you. If you’ve had unexplained side effects from codeine, if you’re planning surgery, if you’re giving it to a child-ask for alternatives. Push for testing. Don’t assume it’s safe because it’s been around for decades.
Final Reality Check
Codeine isn’t evil. It’s just outdated. It was designed before we understood how genes affect drugs. Today, we know better. And we have better tools.
There’s no reason to risk a child’s life because a doctor didn’t think to check a gene. There’s no reason to take a pill that could kill you because it’s "common" or "cheap."
Pharmacogenetics isn’t science fiction. It’s medicine. And it’s here.
Can codeine be safe for children?
For children under 12, codeine is not considered safe under any circumstances. The FDA, Medsafe, and the American Academy of Pediatrics all advise against it due to the risk of fatal respiratory depression in ultrarapid metabolizers. Even in older children, the risk remains unpredictable without genetic testing. Safer alternatives like acetaminophen or ibuprofen should always be used first.
How do I know if I’m an ultrarapid metabolizer?
You can find out through a CYP2D6 genetic test, which is available through most hospital labs and commercial genetic testing companies. The test looks at your DNA to see how many copies of the CYP2D6 gene you have. Results are usually available within 3 to 14 days. If you’ve had unexplained side effects from opioids or have family members who had bad reactions, testing is strongly recommended.
Is genetic testing covered by insurance?
Some insurance plans cover CYP2D6 testing if it’s ordered by a doctor for a specific reason, like prior opioid reactions or planned surgery. Pre-authorization is often required. Costs range from $200 to $500 without insurance. In New Zealand and Australia, public health systems rarely cover it unless part of a research study. Check with your provider before testing.
What are safer painkillers if I’m an ultrarapid metabolizer?
Drugs that don’t rely on CYP2D6 metabolism are safest. These include morphine, hydromorphone, fentanyl, and non-opioid options like acetaminophen or NSAIDs (ibuprofen, naproxen). Avoid tramadol, codeine, hydrocodone, and oxycodone unless you’ve confirmed your metabolism status and a doctor has approved a lower dose.
Why isn’t everyone tested before getting codeine?
Because the healthcare system hasn’t caught up. Testing takes time, costs money, and isn’t yet standard practice in most clinics. Many doctors aren’t trained in pharmacogenetics. Electronic records rarely flag genetic risks. But this is changing. As tests get faster and cheaper, and as more evidence piles up, routine testing will become the norm. Until then, ask questions.


Neil Ellis
Man, this post hit different. I had no idea codeine could turn into a silent killer just because of your DNA. I’m from a mixed background-my mom’s from Nigeria, dad’s Irish-and now I’m wondering if I’m one of those ultrarapid metabolizers. Got my grandma on codeine after knee surgery once… she slept for three days. Thought she was just tired. Now I’m scared.
We need to stop treating medicine like it’s one-size-fits-all. Our bodies aren’t factory models. They’re custom builds. And if we’re gonna call ourselves advanced, we gotta start treating genetics like the basic diagnostic tool it is. Not some fancy add-on.
Also, why is this still not in EHRs? Like, if your blood type pops up, why not your CYP2D6 status? It’s not magic. It’s just… data. We have the tech. We just don’t want to pay for it until someone dies.
And honestly? The fact that it’s still OTC for cough syrup in some places? That’s not negligence. That’s criminal.
Rob Sims
Oh wow, another ‘genetics are magic’ post. Let me guess-you also think we should test everyone for BRCA before they get a cold? This isn’t medicine, it’s sci-fi fanfiction wrapped in a FDA warning.
Codeine’s been around since 1832. People have been using it since the Civil War. And now you want to genetically screen every kid getting their tonsils out? That’s not safety. That’s bureaucratic overreach.
Also, 7% is not ‘high risk.’ It’s a statistical blip. You’re scaring parents into avoiding pain relief because you read a blog post. Chill.
Lauren Wall
My cousin died from codeine after tonsil surgery. They didn’t test her. No one asked. Just gave her the pill. She was 8.
Tatiana Bandurina
It’s fascinating how this post frames genetic variation as a tragedy rather than a biological reality. The real tragedy is that we still treat medicine like a lottery. We don’t screen for CYP2D6 because it’s inconvenient, not because it’s unnecessary. The system doesn’t fail because of ignorance-it fails because of cost-benefit calculations that prioritize profit over prevention.
And yet, the same people who scream about ‘personal responsibility’ in healthcare are the first to blame patients when they overdose on a drug that was never meant for their metabolism.
It’s not about fear. It’s about accountability.
Lana Kabulova
Okay, but… what about the fact that CYP2D6 also affects antidepressants? Like, if you’re ultrarapid, you metabolize SSRIs too fast-so they don’t work. And if you’re poor metabolizer, you get toxicity? So why are we only talking about codeine? Why not talk about the whole damn system? Why are we siloing pharmacogenomics like it’s a niche concern? It’s not. It’s foundational. We’re treating patients like they’re all the same operating system when we know they’re not. And it’s not just codeine-it’s everything. Everything.
And yet, we still don’t have a national pharmacogenomic registry. No one’s incentivized. No one’s responsible. And the patients? They’re the ones who pay with their lives.
It’s not a ‘risk.’ It’s a systemic failure. And it’s been known for over a decade.
Ryan Riesterer
Pharmacogenomic integration remains suboptimal across most clinical environments due to infrastructural and workflow constraints. While the CPIC guidelines are robust, their adoption is hindered by lack of EHR interoperability, insufficient clinician training, and absence of standardized reporting protocols. The 15–20% adoption rate in U.S. hospitals reflects systemic inertia, not scientific ambiguity.
Point-of-care CYP2D6 testing prototypes show promise, but scalability requires regulatory harmonization and reimbursement policy reform. Until then, clinical discretion remains the primary mitigant-despite its documented fallibility.
Daphne Mallari - Tolentino
It is truly lamentable that, in the 21st century, we continue to administer medications without accounting for the most fundamental variable: human genetic variance. The persistence of codeine prescriptions-particularly in pediatric populations-is not merely a lapse in clinical judgment; it is an ethical failure of medical education and institutional governance.
The fact that the FDA issued a boxed warning in 2013, and that the European Medicines Agency banned its use in children under 12 in 2015, yet the drug remains available over the counter in certain jurisdictions, speaks volumes about the dissonance between evidence-based medicine and regulatory pragmatism.
One cannot help but observe that the burden of this negligence falls disproportionately on marginalized communities-those whose ancestral origins place them at higher genetic risk, yet who are least likely to have access to genetic testing or advocacy networks.
It is not enough to say ‘ask your doctor.’ That is a cop-out. The onus must shift from patient to provider. Prescribing codeine without genetic screening should be considered malpractice. It should be flagged in medical records. It should be audited. It should be punished.
We are not talking about theoretical risk. We are talking about children who died because a pill was given without a thought. And we are still giving it.
The solution is not complex: implement mandatory CYP2D6 screening prior to opioid prescription. Integrate results into EMRs. Educate clinicians. Fund public health campaigns. Stop treating genetics as optional.
It is not science fiction. It is science. And we are failing it.
Mike P
Bro, this is why I hate American medicine. We got a drug that’s been used for 200 years and now we’re acting like it’s a bomb because some people got extra genes? I’m Indian-American, my dad’s from Kerala, and he’s been on codeine for back pain since the 90s. He’s fine. My cousin in Delhi takes it for coughs every winter. No one dies. What’s the deal?
You wanna test everyone? Cool. But then why not test for everything? Why not test if you’re gonna get cancer before you eat a burger? We’re turning medicine into a video game where you unlock your DNA skin and then you get the safe painkiller skin. It’s ridiculous.
And don’t even get me started on the ‘genetic testing is expensive’ crowd. I paid $400 for a 23andMe kit. That’s cheaper than a damn MRI. Just do it. Stop whining.
Sarvesh CK
It is worth contemplating, in the grand tapestry of medical progress, that the human body has evolved over millennia with remarkable adaptability-yet our pharmacological interventions remain stubbornly static, rooted in a paradigm that assumes homogeneity where none exists. The CYP2D6 polymorphism is not an anomaly; it is a natural variation, as integral to human diversity as skin tone or blood type. To ignore it is to treat medicine as an art of guesswork rather than a science of precision.
Moreover, the reluctance to implement widespread genetic screening is not merely a matter of cost or logistics-it is a reflection of deeper epistemological inertia. We are conditioned to believe that ‘common’ equals ‘safe,’ and ‘established’ equals ‘reliable.’ But history is replete with examples where the familiar became the fatal: thalidomide, DES, Vioxx. Each time, we were told the same thing: ‘It’s been used for decades.’
The path forward lies not in fear, but in humility. We must acknowledge that our understanding of physiology is incomplete, and that the most compassionate care is not the most convenient, but the most individualized. A single pill, prescribed without context, is not care-it is chance.
Perhaps the true revolution in medicine is not in the discovery of new drugs, but in the willingness to see each patient not as a statistic, but as a unique constellation of genes, history, and biology.
Let us not wait for another child to die before we choose to see them as human.
Akriti Jain
Let me guess… the pharmaceutical companies are behind this ‘genetic testing’ push. Why now? Because they just made a new painkiller that costs $500 a pill and doesn’t need testing. Classic. They’re using fear to sell you a more expensive product. Codeine is cheap. They want you to switch. They don’t care about your kids-they care about your credit card.
And who’s running these genetic labs? Big Pharma. Who’s funding the ‘experts’? Big Pharma. The FDA? They’re on the payroll. You think they’d ban codeine if it didn’t make someone else richer?
Also, why is this only a problem in the US and Europe? Why not in India or Africa? Oh right… because they don’t have the money to test. So it’s a rich person’s problem. Classic.
Don’t fall for it. Stick with the old stuff. The system wants you scared. Don’t give them the satisfaction.
arun mehta
Dear friends, this is not just about codeine-it is about the soul of modern medicine. 🙏
When we prescribe without knowing the genetic blueprint of the patient, we are not healing-we are gambling. And every time we do it, we risk the life of a child who trusted us.
India, too, has ultrarapid metabolizers-especially in South Indian populations. Yet, codeine remains freely available in pharmacies, often without prescription.
Let us not wait for tragedy to strike before we act. Let us demand, with calm urgency, that our hospitals adopt pharmacogenomic screening as a standard of care.
It is not expensive. It is not futuristic. It is simply… right.
And if you are reading this, please-ask your doctor. Ask your pharmacist. Ask your child’s pediatrician.
Knowledge is not fear. Knowledge is love in action. 💙
- With hope, Arun
Kenji Gaerlan
wait so u mean i coulda died from a cough syrup?? like… im 24 and i took that stuff 3 times last winter… is it too late??
also my mom gave it to my 5yo cousin after his vaccine… she said it was ‘just a little something to calm him’… oh god
Keith Helm
While the clinical rationale for avoiding codeine in ultrarapid metabolizers is well established, the implementation of preemptive pharmacogenetic testing remains logistically unfeasible at scale. The cost-benefit analysis does not currently justify population-wide screening. Targeted testing-based on clinical indicators such as prior opioid intolerance or family history of adverse reactions-remains the most pragmatic approach.
Furthermore, the variability in CYP2D6 activity across ethnic populations does not equate to deterministic risk. Environmental factors, drug interactions, and comorbidities significantly modulate metabolic outcomes.
Policy recommendations must be grounded in feasibility, not idealism.
Alec Amiri
Okay, but… why is no one talking about the fact that this is happening because doctors are lazy? They don’t wanna look up guidelines. They don’t wanna read a 20-page PDF. They just grab the first thing on the formulary and call it a day.
And guess what? The patients? They’re the ones who get the pill. And if they die? Well… it’s ‘an unfortunate complication.’
This isn’t a genetics problem. It’s a culture problem. A ‘we’ve always done it this way’ culture. And until we start holding doctors accountable for not knowing their own damn guidelines, nothing’s gonna change.
Also, if you’re giving codeine to a kid after tonsil surgery? You’re not a doctor. You’re a liability.
Daphne Mallari - Tolentino
It is not enough to say ‘we know better.’ We must act as if we know better. The fact that this information has been available since 2012-and yet, in 2024, codeine is still prescribed without genetic screening-is not a failure of technology. It is a failure of moral courage.
Every time a clinician writes a prescription without considering pharmacogenomics, they are choosing convenience over consequence. They are choosing the path of least resistance over the path of greatest responsibility.
And when a child dies? We do not call it negligence. We call it ‘an unfortunate outcome.’
That is the language of complicity.