Medications with a Narrow Therapeutic Index: Why Expired Drugs Are Dangerous
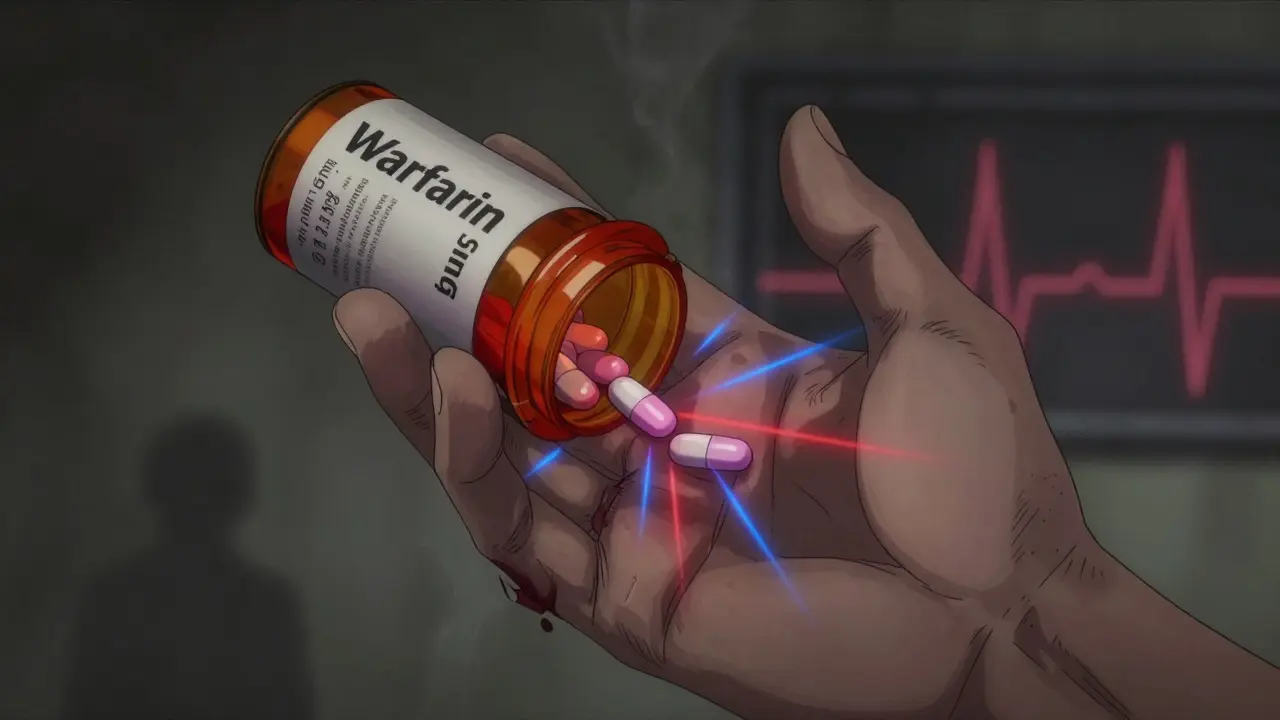
When a medication says "expires on 12/2025," it doesn’t mean it suddenly turns toxic on December 1st. But for some drugs, even a tiny drop in potency after that date can be life-threatening. This isn’t about old aspirin losing its punch. It’s about drugs where narrow therapeutic index means there’s almost no room for error-between helping you and harming you.
What Exactly Is a Narrow Therapeutic Index?
A narrow therapeutic index (NTI) drug is one where the difference between a safe, effective dose and a dangerous, toxic dose is razor-thin. Think of it like walking a tightrope blindfolded. One misstep, and you fall. The U.S. Food and Drug Administration defines NTI drugs as those where small changes in blood concentration can cause serious harm-like internal bleeding, seizures, heart failure, or even death.
For most medications, a 10% drop in potency might just mean it works a little slower. For NTI drugs, that same 10% could push you from the therapeutic range into the toxic zone. Take digoxin, used for heart rhythm problems. The safe level in your blood is between 0.5 and 0.9 nanograms per milliliter. Toxicity starts above 1.2. That’s only a 33% jump from the top of the safe range to the danger zone. If your pill loses even 5% of its strength due to age or heat, your body might not get enough. But if it degrades unevenly and concentrates in some parts of the tablet? You could get too much-and that’s just as dangerous.
Common NTI Medications You Might Be Taking
You might not realize you’re on an NTI drug. These aren’t obscure chemicals-they’re widely prescribed. Here are the most common ones:
- Warfarin (Coumadin, Jantoven): Used to prevent blood clots. A 10% dose change can shift your INR (a blood test measuring clotting time) by 0.5 to 1.0. If your INR goes above 4, you’re at high risk of uncontrolled bleeding.
- Lithium: For bipolar disorder. Blood levels must stay between 0.6 and 1.0 mmol/L. Above 1.5, you risk tremors, confusion, kidney damage, or coma.
- Digoxin: For heart failure and atrial fibrillation. As mentioned, the margin between effective and toxic is tiny.
- Phenytoin and Carbamazepine: Antiseizure drugs. Even slight drops in blood levels can trigger breakthrough seizures.
- Levothyroxine: For hypothyroidism. A small change can throw off your metabolism, heart rate, and energy levels for weeks.
- Ciclosporin and Tacrolimus: Immunosuppressants after organ transplants. Too little? Rejection. Too much? Kidney failure.
These aren’t optional meds. They’re not "nice to have." They’re essential for survival or preventing irreversible damage. And their effectiveness depends on precision-not guesswork.
Why Expiration Dates Matter More for NTI Drugs
Drug expiration dates aren’t arbitrary. They’re based on real stability testing. Manufacturers test how long a drug keeps at least 90% of its labeled potency under recommended storage conditions (cool, dry, out of sunlight). For most pills, 90% potency is still safe and effective. For NTI drugs? That 10% loss could be the difference between control and crisis.
Take warfarin. If your prescription is supposed to deliver 5 mg per tablet, and it degrades to 4.5 mg after expiration, that’s a 10% drop. For someone on warfarin, that’s enough to make their INR drop from 2.8 to 2.3. Sounds minor? Maybe. But if you have a mechanical heart valve, your target INR is 2.5-3.5. Falling below 2.5 means your blood starts clotting again. You could develop a stroke or pulmonary embolism without warning.
And it’s not just about losing strength. Some drugs break down into harmful byproducts. Tetracycline, for example, turns toxic when expired. While not an NTI drug, it shows degradation isn’t always harmless. For NTI drugs, we don’t have enough data on exactly what their degradation products do-but we know even small changes in concentration can trigger reactions. So we assume the worst.

How Regulators Handle NTI Drugs-And Why It’s Not Enough
The FDA treats NTI drugs differently than others. Since 2011, generic versions of NTI drugs must meet tighter bioequivalence standards: 90-111% of the brand-name drug’s absorption, instead of the usual 80-125%. That means if you switch from one generic to another, the difference in how your body absorbs it can’t be more than 11%. That’s strict.
But here’s the gap: no one has studied what happens when these drugs go past their expiration date. The FDA says most drugs remain potent for years beyond expiration. That’s true for ibuprofen or amoxicillin. But for NTI drugs? There’s no data. No studies. No guidelines. Just silence.
Pharmaceutical companies now do extended stability testing on many NTI drugs-78% of major manufacturers, according to Pharmaceutical Technology in 2022. But they don’t publish those results. They don’t extend expiration dates. They don’t warn patients. So you’re left guessing.
What You Should Do If You’re on an NTI Drug
Don’t wait for a recall. Don’t hope it’s still okay. Here’s what works in real life:
- Check expiration dates every time you refill. Don’t assume the pharmacy gave you a fresh batch. If the date is past, ask for a new one. No argument. No "it’s probably fine."
- Store them properly. Keep them in a cool, dry place-not the bathroom medicine cabinet. Heat and humidity speed up degradation. A bedroom drawer is better.
- Never use leftover pills. If your doctor changed your dose, don’t save the old ones. Even if they’re not expired, they’re not the right strength anymore.
- Ask your pharmacist about brand vs. generic. Some NTI drugs, like levothyroxine, have known differences between brands. Stick with one manufacturer unless your doctor says otherwise.
- Get regular blood tests. If you’re on warfarin, lithium, or digoxin, you should be monitored regularly. If your levels are fluctuating without a reason, expired or degraded medication could be why.
Pharmacists in North Carolina and other states are trained to flag NTI drugs at pickup. If your pharmacist doesn’t mention it, ask: "Is this one of those drugs where the dose has to be exact?" If they hesitate, get a second opinion.

What Happens When People Ignore Expiration Dates
There are no big headlines about people dying from expired NTI drugs-because most cases go unreported. But the clinical evidence is clear. A 2014 study in the Journal of Clinical Pharmacy and Therapeutics found that NTI drugs were far more likely than others to cause serious adverse reactions due to dosing errors. That includes accidental overdoses from switching brands, but also underdosing from degraded pills.
One real case from a New Zealand hospital in 2023 involved an elderly woman on digoxin. She’d been using the same bottle for 18 months past expiration. Her heart rate dropped dangerously low. Blood tests showed her digoxin level was half what it should be. She’d been taking the same number of pills-but the drug had degraded. She spent a week in ICU. Her family didn’t know expiration mattered for heart meds.
That’s the problem. Most people think expiration dates are about taste or color. They’re not. For NTI drugs, they’re about life or death.
What’s Being Done-and What’s Not
The American Pharmacists Association called in 2021 for special labeling on NTI drugs: "Do not use after expiration. Potency critical." No such labels exist yet. The European Medicines Agency recognizes the risk but hasn’t acted. The FDA has guidelines for how generics must match brand-name drugs-but nothing for what happens after the bottle is opened and left on a shelf for years.
Until that changes, the burden falls on you. If you’re taking one of these drugs, treat it like insulin or epinephrine. If it’s expired, replace it. No exceptions. No "I’ll use it until the next refill." Your body doesn’t have a buffer. Neither should you.
Can I still take an NTI drug if it’s a few months past its expiration date?
No. Even a few months past expiration can mean a 5-10% loss in potency for NTI drugs like warfarin, lithium, or digoxin. That small change can push your blood levels outside the safe range, leading to serious complications like blood clots, seizures, or toxicity. Never use expired NTI medications-replace them immediately.
Are generic NTI drugs safe to use?
Yes-but only if they meet strict bioequivalence standards. The FDA requires generic NTI drugs to be within 90-111% of the brand-name drug’s absorption, compared to 80-125% for most drugs. However, switching between different generic brands can still cause fluctuations. Stick with the same manufacturer if possible, and always monitor your blood levels closely.
How do I know if my medication has a narrow therapeutic index?
Ask your pharmacist or doctor directly. Common NTI drugs include warfarin, lithium, digoxin, phenytoin, levothyroxine, carbamazepine, and ciclosporin. If your doctor says you need regular blood tests to monitor your levels, you’re likely on an NTI drug. Never assume-confirm it.
Can expired NTI drugs become toxic?
While most NTI drugs lose potency rather than turn toxic, some degradation products can be harmful. For example, expired tetracycline forms toxic compounds. Though not an NTI drug, this shows degradation isn’t always harmless. For NTI drugs, we lack data-but we know even minor concentration changes can be dangerous. The safest choice is always to discard expired ones.
Should I keep extra NTI medications on hand?
Only if you can rotate them properly. Don’t stockpile. If you have extra, use the oldest first. Never rely on pills that are more than 6 months past expiration, even if you haven’t opened them. NTI drugs are too critical to risk. Always get fresh prescriptions before your supply runs out.
If you’re on an NTI drug, your life depends on consistency. Consistent dose. Consistent storage. Consistent expiration dates. Don’t gamble with your health. When the margin for error is this small, the only safe choice is to never use an expired pill.



Dominic Suyo
Let’s be real - this isn’t about expiration dates. It’s about Big Pharma’s profit model. They don’t want you to know that most NTI drugs are stable for years. Why? Because if you didn’t have to refill every 30 days, their quarterly earnings would crater. That’s why they slap on arbitrary dates and scare you with ‘toxicity’ myths. Digoxin doesn’t turn into VX nerve agent after Dec 1st. It just gets weaker. And guess what? Your doctor already accounts for variability. You’re being manipulated.
Kevin Motta Top
My grandma took warfarin for 12 years past expiration. Never had a clot. Never bled out. She stored it in a drawer. No humidity. No sun. The system is broken - but not because of the pills.
Alisa Silvia Bila
I’m a nurse and I’ve seen both sides. Expired NTI meds? I’d never risk it. But I’ve also seen people who can’t afford replacements and end up skipping doses - which is way more dangerous. We need better access, not just fear.
Henry Marcus
EVERY SINGLE EXP DATE IS A LIE. THE FDA IS IN BED WITH PHARMA. THEY’RE LYING TO YOU ABOUT STABILITY. THEY KNOW THE DRUGS LAST YEARS - BUT THEY’RE PROFITING OFF YOUR FEAR. THEY’RE SELLING YOU NEW BOTTLES LIKE THEY’RE FRESH MILK. THE TRUTH? THEY’RE ALL STILL GOOD. YOU’RE BEING ROBBED. OPEN YOUR EYES.
Carolyn Benson
There’s a metaphysical layer here. The expiration date isn’t just chemical - it’s symbolic. It represents our surrender to institutional authority. We outsource our bodily autonomy to a label printed by a corporation that doesn’t care if we live or die. We’ve been conditioned to believe that time, not our own intuition, governs our survival. And that’s the real toxicity.
Aadil Munshi
Bro, you’re overcomplicating this. NTI drugs? Yeah, they’re sensitive. But the real issue? Pharmacies don’t rotate stock. You get the oldest batch first - sometimes months past expiration. And no one tells you. I work in a pharmacy in Bangalore. We’ve had warfarin tablets from 2021 still sitting on shelves. Patients? They don’t know. The system’s broken, not the meds.
Kinnaird Lynsey
I get the concern. But I also know people who’ve been on lithium for 20 years and still use the same bottle. They monitor their levels religiously. Maybe the answer isn’t throwing them out - it’s better monitoring and education. Not fear.
mark shortus
OMG I JUST REALIZED MY DIGOXIN IS EXPIRED 😭 I’M GONNA DIE I’M SURE OF IT I’M SENDING THIS TO MY DOCTOR RIGHT NOW AND I’M GOING TO THE ER AND THEY’RE GOING TO PUT ME ON A MONITOR AND I’M NEVER TAKING A PILL AGAIN WITHOUT A BARCODE SCANNER AND A LAB ANALYSIS AND A THERAPIST AND A BLESSING FROM A SHAMAN
Elaine Douglass
I’m on levothyroxine and I used to panic every time my script ran out. Then I started checking my labs every 3 months. Turns out, my body’s pretty good at adapting. I still replace expired meds - but I don’t lose sleep over it. Your doctor’s your ally, not your enemy.
Emily P
Is there a database somewhere that lists which generics are bioequivalent for NTI drugs? I’ve been switching brands and wondering if that’s why my TSH is all over the place.
Vicki Belcher
Thank you for this!! 💖 I’m on warfarin and I had no idea expiration mattered this much. Just got my refill and checked the date - it’s good! I’ll keep my meds in my bedroom now 😊 You’re a lifesaver!
Aboobakar Muhammedali
My cousin in Delhi was on tacrolimus after transplant. He used expired pills because he couldn’t afford new ones. He got rejection. He didn’t die - but he lost the kidney. This isn’t theoretical. It’s real. And it’s happening every day in places no one talks about.
Laura Hamill
THIS IS ALL A CULTURE WAR. THE LEFT WANTS YOU TO BE AFRAID OF PILLS. THEY WANT YOU TO DEPEND ON DOCTORS AND PHARMACIES. THEY’RE USING NTI DRUGS TO CONTROL YOU. THE GOVERNMENT KNOWS THEY LAST YEARS. THEY’RE HIDING IT. DON’T BE A SHEEP.
Alana Koerts
Stop dramatizing. 90% potency is fine for NTI drugs. The FDA’s 10% buffer is conservative. The real problem? Poor adherence. People forget doses. They take them with grapefruit. They switch brands. Not expiration. Stop blaming the date.
pascal pantel
Let me break this down with actual pharmacokinetics. For warfarin, the half-life is 36-42 hours. A 10% potency loss over 6 months? That’s 0.000002% daily degradation. The variability in absorption from food, gut flora, CYP2C9 polymorphisms, and liver enzyme induction dwarfs that. You’re more likely to die from eating kale than from degraded warfarin. This is fearmongering dressed as science.